How To Make Orbital Diagrams
Electron configuration orbital diagram drawing diagrams configurations atoms aufbau arrangement rules example Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level libretexts cl2 delocalized second electron homonuclear row Orbital electron diagrams configuration diagram potassium atom 2s configurations 1s 3s 2p ppt powerpoint presentation slideserve there
Orbital | Chemistry, Physics & Applications | Britannica
Chapter 8 section b quantum numbers for electrons Orbitals shapes draw shape 3d chemistry orbital five electron drawings four these Molecular orbital theory
Orbitals electron electronic single orbital atomic shapes nodes electrons quantum diagram atom chemistry orbitales chemwiki radial atoms structure diagrams there
Orbital diagrams and electron configurationOrbital diagrams orbitals electrons overview monahan Orbital diagrams — overview & examplesHow do you draw s,p,d,f orbitals?.
Orbitals atomic molecular molecules internuclear takingElectron configuration orbital table periodic order chart config electrons configurations per shells following use just Electron configurations and atomic orbital diagramsOrbital diagrams — overview & examples.

Orbital diagrams monahan
Electron configurationsOrbital orbitals subshell symmetry socratic Which are the orbitals(s,p,d,f) have center of symmetry?Orbitals shapes atomic chemistry atoms chem quantum electrons shape electron atom numbers wave model chart theory cartesian orbital diagram energy.
Solved you can ignore the principle quantum number nDrawing electron configurations with aufbau/orbital diagram 6.6: 3d representation of orbitalsOrbitals atomic molecular orbital quantum number between difference px draw 2p 3d overlap principle 3dz atoms axes each coordinate ignore.

Electron configuration orbital atomic electrons valence transition configurations metals phosphorus orbitals elements element chemistry diagrams level diagram state number atom
Orbital molecular theory n2 orbitals diatomic valence o2 atomic carbon homonuclear sp3 molecule majors cnx chem atomsElectronic pairing structure orbital diagrams chemistry quantum diagram spin notation box electrons electron orbitals energy first boxes spins configurations level Orbital diagramsOrbital electron orbitals atoms dimensional depicted.
Drawing atomic and molecular orbitals diagrams for moleculesOrbitals 3d representation chemistry chem libretexts electronic figure 6.6: the shapes of atomic orbitalsElectronic orbitals.

Molecular orbital theory
Orbital electron diagrams configuration chemistry practice problems basicOrbitals electron orbital orbitali electrons quantum atomici atomic quantici numeri biopills atom atoms libretexts chimica arrangement directional toppr atomo nscc .
.


Solved you can ignore the principle quantum number n | Chegg.com

Molecular Orbital Theory - Chemistry LibreTexts

Orbital Diagrams — Overview & Examples - Expii

6.6: The Shapes of Atomic Orbitals - Chemistry LibreTexts
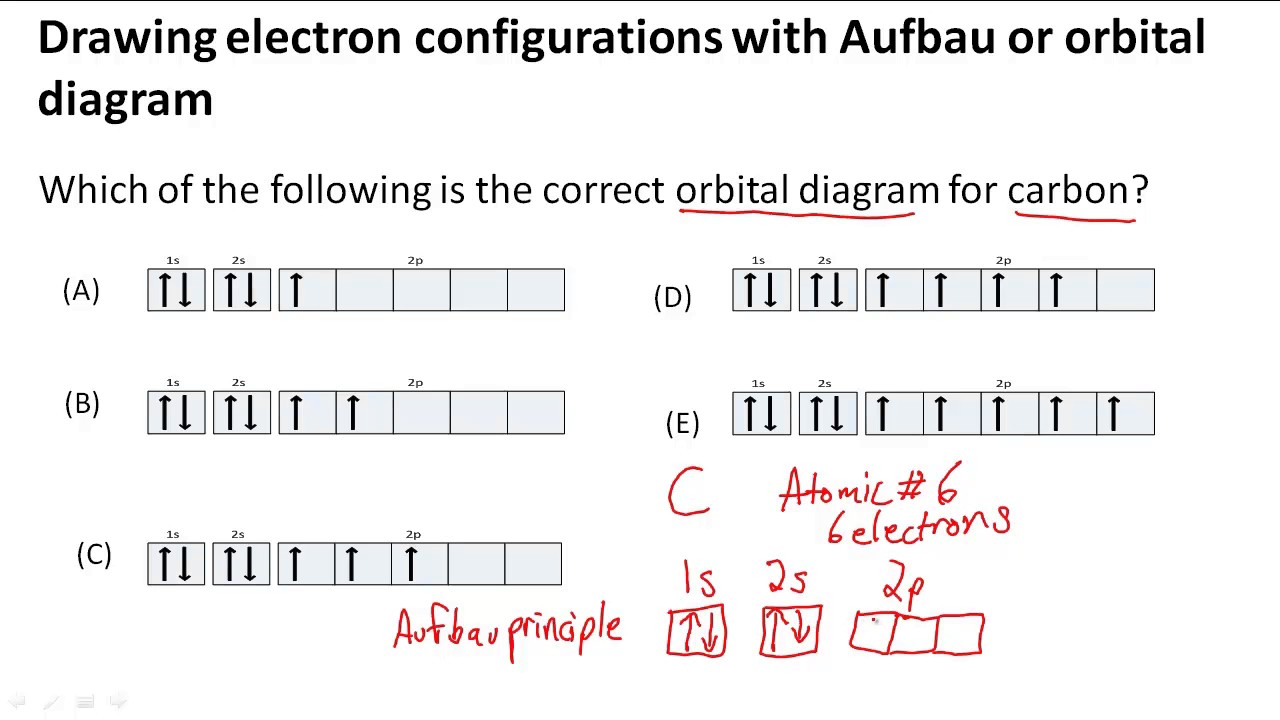
Drawing electron configurations with Aufbau/orbital diagram - YouTube

Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic

Electron Configurations and Atomic Orbital Diagrams - Chemistry

Orbital | Chemistry, Physics & Applications | Britannica